The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex.[1][2] The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination complexes; crystal field theory and ligand field theory, which is a more advanced version based on molecular orbital theory.[3]
- Phosphorus Number Of Neutron
- Phosphorus Number Of Electrons To Lose
- Phosphorus Number Of Electrons Gained Or Lost
- Phosphorus Number Of Electrons And Protons
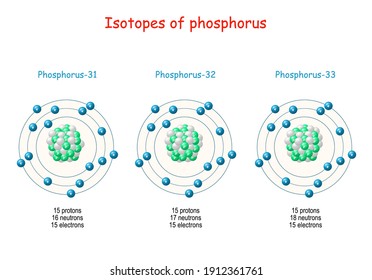
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram (compare copper at about. The atomic number of phosphorus is 15. That means the electronic configuration is 2:8:5. That means this element needs to gain 3 electrons to become a anion(negitive ion). Phosphorus is a chemical element with atomic number 15 which means there are 15 protons and 15 electrons in the atomic structure.
Standard electron configuration perspective[edit]
The electron configuration for transition metals predicted by the simple Aufbau principle and Madelung's rule has serious conflicts with experimental observations for transition metal centers under most ambient conditions. Under most conditions all of the valence electrons of a transition metal center are located in d orbitals while the standard model of electron configuration would predict some of them to be in the pertinent s orbital.
Electrons have a specific form of distribution (or configuration) in every atom, even Phosphorus. Some are hard to memorise (or predict), so what is the electron configuration of an atom of P? In the case of Phosphorus the abbreviated electron configuration is Ne 3s2 3p3. Nevertheless, check the complete configuration and other interesting. Phophorus is in group 15, it has 5 electrons in its outer shell, 3s23p3. When it forms chemical compounds it can share electrons to form covalent bonds or gain 3 electrons to form the P3- phosphide. How to get addons tauriwow.
The valence of a transition metal center can be described by standard quantum numbers. The Aufbau principle and Madelung's rule would predict for period n that the ns orbitals fill prior to the (n − 1)d orbitals. For example, the 4s fills before the 3d in period 4. In general chemistry textbooks, a few exceptions are acknowledged with only one electron in the ns orbital in favor of completing a half or whole d shell. The usual explanation is that 'half-filled or completely filled subshells are particularly stable arrangements of electrons'. An example is chromium whose electron configuration is [Ar]4s13d5 with a half-filled d subshell, although Madelung's rule would predict [Ar]4s23d4. Similarly copper is [Ar]4s13d10 with a full d subshell, and not [Ar]4s23d9.[3]:38
Matters are further complicated when metal centers are oxidized. Since the (n − 1)d shell is predicted to have higher energy than the ns shell, it might be expected that electrons would be removed from the (n − 1)d shell first. Experimentally it has been observed that not only are the ns electrons removed first, even for unionized complexes all of the valence electrons are located in the (n − 1)d orbitals.
There are various hand waving arguments for this phenomenon including that 'the ns electrons are farther away from the nuclei and thus ionized first' while ignoring results based on neutral complexes. This poor explanation avoids the basic problems with the standard electron configuration model. The standard electron configuration model assumes a hydrogen-like atom removed from all other atoms. This assumption is only truly relevant for esoteric situations. It is far more common for metal centers to have bonds to other atoms through metallic bonds or covalent bonds. These bonds drastically change the energies of the orbitals for which electron configurations are predicted. Thus for coordination complexes the standard electron configuration formalism is meaningless and the d electron count formalism is a suitable substitute.
Ligand field perspective[edit]
Crystal field theory describes a number of physical phenomena well but does not describe bonding nor offer an explanation for why ns electrons are ionized before (n − 1)d electrons. The more recent ligand field theory offers an easy to understand explanation that models phenomena relatively well.
According to the model present by ligand field theory, the ns orbital is involved in bonding to the ligands and forms a strongly bonding orbital which has predominantly ligand character and the correspondingly strong anti-bonding orbital which is unfilled and usually well above the lowest unoccupied molecular orbital (LUMO). Since the orbitals resulting from the ns orbital are either buried in bonding or elevated well above the valence, the ns orbitals are not relevant to describing the valence. Depending on the geometry of the final complex, either all three of the np orbitals or portions of them are involved in bonding, similar to the ns orbitals. The np orbitals if any that remain non-bonding still exceed the valence of the complex. That leaves the (n − 1)d orbitals to be involved in some portion of the bonding and in the process also describes the metal complex's valence electrons. The final description of the valence is highly dependent on the complex's geometry, in turn highly dependent on the d electron count and character of the associated ligands.
For example, in the MO diagram provided for the [Ti(H2O)6]3+ the ns orbital – which is placed above (n − 1)d in the representation of atomic orbitals (AOs) – is used in a linear combination with the ligand orbitals, forming a very stable bonding orbital with significant ligand character as well as an unoccupied high energy antibonding orbital which is not shown. In this situation the complex geometry is octahedral, which means two of the d orbitals have the proper geometry to be involved in bonding. The other three d orbitals in the basic model do not have significant interactions with the ligands and remain as three degenerate non-bonding orbitals. The two orbitals that are involved in bonding form a linear combination with two ligand orbitals with the proper symmetry. This results in two filled bonding orbitals and two orbitals which are usually the lowest unoccupied molecular orbitals (LUMO) or the highest partially filled molecular orbitals – a variation on the highest occupied molecular orbitals (HOMO).
Tanabe–Sugano diagram[edit]
Each of the ten possible d electron counts has an associated Tanabe–Sugano diagram describing gradations of possible ligand field environments a metal center could experience in an octahedral geometry. The Tanabe–Sugano diagram with a small amount of information accurately predicts absorptions in the UV and visible electromagnetic spectrum resulting from d to d orbital electron transitions. It is these d–d transitions, ligand to metal charge transfers (LMCT), or metal to ligand charge transfers (MLCT) that generally give metals complexes their vibrant colors.
Limitation[edit]
It is important to remember that the d electron count is a formalism and describes some complexes better than others. Often it is difficult or impossible to assign electrons and charge to the metal center or a ligand. For a high-oxidation-state metal center with a +4 charge or greater it is understood that the true charge separation is much smaller. But referring to the formal oxidation state and d electron count can still be useful when trying to understand the chemistry.
Possible d electron counts[edit]
There are many examples of every possible d electron configuration. What follows is a short description of common geometries and characteristics of each possible d electron count and representative examples.
- d0
- Commonly tetrahedral; however it is possible for d0 complexes to accommodate many electron pairs (bonds/coordination number) since their d orbitals are empty and well away from the 18-electron ceiling. Often colorless due to the lack of d to d transitions.
- Examples: titanium tetrachloride, titanocene dichloride, Schwartz's reagent.
- d1
- Examples: molybdenum(V) chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium tetrachloride.
- d2
- Examples: titanocene dicarbonyl.
- d3
- Examples: Reinecke's salt.
- d4
- Octahedral high-spin: 4 unpaired electrons, paramagnetic, substitutionally labile.
- Octahedral low-spin: 2 unpaired electrons, paramagnetic, substitutionally inert.
- d5
Phosphorus Number Of Neutron
- Octahedral high-spin: 5 unpaired electrons, paramagnetic, substitutionally labile.
- Octahedral low-spin: 1 unpaired electron, paramagnetic, substitutionally inert.
- Examples: potassium ferrioxalate, vanadium carbonyl.
- d6
- Commonly octahedral complexes in both high spin and low spin.
- Octahedral high-spin: 4 unpaired electrons, paramagnetic, substitutionally labile.
- Octahedral low-spin: no unpaired electrons, diamagnetic, substitutionally inert.
- Examples: hexamminecobalt(III) chloride, sodium cobaltinitrite, molybdenum hexacarbonyl, ferrocene, ferroin, chromium carbonyl.
- d7
- Octahedral high spin: 3 unpaired electrons, paramagnetic, substitutionally labile.
- Octahedral low spin: 1 unpaired electron, paramagnetic, substitutionally labile.
- Examples: cobaltocene.
- d8
- Complexes which are d8 high-spin are usually octahedral (or tetrahedral) while low-spin d8 complexes are generally 16-electron square planar complexes. For first row transition metal complexes such as Ni2+ and Cu+ also form five-coordinate 18-electron species which vary from square pyramidal to trigonal bipyramidal.
- Octahedral high spin: 2 unpaired electrons, paramagnetic, substitutionally labile.
- Square planar low spin: no unpaired electrons, diamagnetic, substitutionally inert.
- Examples: cisplatin, nickelocene, dichlorobis(ethylenediamine)nickel(II), iron pentacarbonyl, Zeise's salt, Vaska's complex, Wilkinson's catalyst.
- d9
- Stable complexes with this electron count are more common for first row (period four) transition metals center than they are for complexes based around second or third row transition metals centers. These include both four-coordinate 17-electron species and five-coordinate 19-electron species.
- Examples: Schweizer's reagent.
- d10
- Often tetrahedral complexes limited to form 4 additional bonds (8 additional electrons) by the 18-electron ceiling. Often colorless due to the lack of d to d transitions.
- Examples: tetrakis(triphenylphosphine)palladium(0), nickel carbonyl.
References[edit]
- ^Green, Malcolm L. H. (1995-09-20). 'A new approach to the formal classification of covalent compounds of the elements'. Journal of Organometallic Chemistry. 500 (1–2): 127–148. doi:10.1016/0022-328X(95)00508-N. ISSN0022-328X.
- ^MLX Plots (Ged Parkin group website, Columbia University)
- ^ abMiessler, Gary L.; Tarr, Donald A. (1998). Inorganic Chemistry (2nd ed.). Upper Saddle River, NJ: Pearson Education. ISBN0-13-841891-8.
External links[edit]
- Pavarini, E.; Koch, E.; Anders, F.; Jarrell, M., eds. (2012). 'Multiplets in Transition Metal Ions'. Correlated Electrons: From Models to Materials(PDF). Jülich. ISBN978-3-89336-796-2.
The Chemistry of Nitrogen and Phosphorous
| The Chemistry of Nitrogen | The Synthesis of Ammonia | The Synthesis of Nitric Acid |
| Intermediate Oxidation Numbers | Negative Oxidation Numbers of Nitrogen Besides -3 | Positive Oxidation Numbers for Nitrogen: The Nitrogen Halides |
| Positive Oxidation Numbers for Nitrogen: The Nitrogen Oxides | The Chemistry of Phosphorus | The Effect of the Differences in the Singe and Triple Bond Strengths |
| The Effect of Differences in the Strengths of P=X and N=X Double Bonds | The Effect of Differences in the Electronegativities of Phosphorus and Nitrogen | The Effect of Differences in the Abilities of Phosphorus and Nitrogen to Expand Their Valence Shell |
The chemistry of nitrogen is dominated by the ease with which nitrogen atoms form double and triple bonds. A neutral nitrogen atom contains five valence electrons: 2s2 2p3. A nitrogen atom can therefore achieve an octet of valence electrons by sharing three pairs of electrons with another nitrogen atom.
Because the covalent radius of a nitrogen atom is relatively small (only 0.070 nm), nitrogen atoms come close enough together to form very strong bonds. The bond-dissociation enthalpy for the nitrogen-nitrogen triple bond is 946 kJ/mol, almost twice as large as that for an O=O double bond.
The strength of the nitrogen-nitrogen triple bond makes the N2 molecule very unreactive. N2 is so inert that lithium is one of the few elements with which it reacts at room temperature.
In spite of the fact that the N2 molecule is unreactive, compounds containing nitrogen exist for virtually every element in the periodic table except those in Group VIIIA (He, Ne, Ar, and so on). This can be explained in two ways. First, N2 becomes significantly more reactive as the temperature increases. At high temperatures, nitrogen reacts with hydrogen to form ammonia and with oxygen to form nitrogen oxide.
| N2(g) | + | 3 H2(g) | 2 NH3(g) |
| N2(g) | + | O2(g) | 2 NO(g) |
Second, a number of catalysts found in nature overcome the inertness of N2 at low temperature.
It is difficult to imagine a living system that does not contain nitrogen, which is an essential component of the proteins, nucleic acids, vitamins, and hormones that make life possible. Animals pick up the nitrogen they need from the plants or other animals in their diet. Plants have to pick up their nitrogen from the soil, or absorb it as N2 from the atmosphere. The concentration of nitrogen in the soil is fairly small, so the process by which plants reduce N2 to NH3 or 'fix' N2 is extremely important.
Although 200 million tons of NH3 are produced by nitrogen fixation each year, plants, by themselves, cannot reduce N2 to NH3. This reaction is carried out by blue-green algae and bacteria that are associated with certain plants. The best-understood example of nitrogen fixation involves the rhizobium bacteria found in the root nodules of legumes such as clover, peas and beans. These bacteria contain a nitrogenase enzyme, which is capable of the remarkable feat of reducing N2 from the atmosphere to NH3 at room temperature.
Ammonia is made on an industrial scale by a process first developed between 1909 and 1913 by Fritz Haber. In the Haber process, a mixture of N2 and H2 gas at 200 to 300 atm and 400 to 600oC is passed over a catalyst of finely divided iron metal.
Almost 20 million tons of NH3 are produced in the United States each year by this process. About 80% of it, worth more than $2 billion, is used to make fertilizers for plants that can't fix nitrogen from the atmosphere. On the basis of weight, ammonia is the second most important industrial chemical in the United States. (Only sulfuric acid is produced in larger quantities.)
Two-thirds of the ammonia used for fertilizers is converted into solids such as ammonium nitrate, NH4NO3; ammonium phosphate, (NH4)3PO4; ammonium sulfate, (NH4)2SO4; and urea, H2NCONH2. The other third is applied directly to the soil as anhydrous (literally, 'without water') ammonia. Ammonia is a gas at room temperature. It can be handled as a liquid when dissolved in water to form an aqueous solution. Alternatively, it can be cooled to temperatures below -33oC, in which case the gas condenses to form the anhydrous liquid, NH3(l).
The NH3 produced by the Haber process that is not used as fertilizer is burned in oxygen to generate nitrogen oxide.
| 4 NH3(g) | + | 5 O2(g) | 4 NO(g) | + | 6 H2O(g) |
Nitrogen oxideor nitric oxide, as it was once known is a colorless gas that reacts rapidly with oxygen to produce nitrogen dioxide, a dark brown gas.

Nitrogen dioxide dissolves in water to give nitric acid and NO, which can be captured and recycled.
| 3 NO2(g) | + | H2O(l) | 2 HNO3(aq) | + | NO(g) |
Thus, by a three-step process developed by Friedrich Ostwald in 1908, ammonia can be converted into nitric acid.
| 4 NH3(g) | + | 5 O2(g) | 4 NO(g) | + | 6 H2O(g) |
| 2 NO(g) | + | O2(g) | 2 NO2(g) | ||
| 3 NO2(g) | + | H2O(l) | 2 HNO3(aq) | + | NO(g) |
The Haber process for the synthesis of ammonia combined with the Ostwald process for the conversion of ammonia into nitric acid revolutionized the explosives industry. Nitrates have been important explosives ever since Friar Roger Bacon mixed sulfur, saltpeter, and powdered carbon to make gunpowder in 1245.
| 16 KNO3(s) | + | S8(s) | + | 24 C(s) | 8 K2S(s) | + | 24 CO2(g) | + | 8 N2(g) | Ho = -571.9 kJ/mol N2 |
Pytivo vs kmttg for mac. Before the Ostwald process was developed the only source of nitrates for use in explosives was naturally occurring minerals such as saltpeter, which is a mixture of NaNO3 and KNO3. Once a dependable supply of nitric acid became available from the Ostwald process, a number of nitrates could be made for use as explosives. Combining NH3 from the Haber process with HNO3 from the Ostwald process, for example, gives ammonium nitrate, which is both an excellent fertilizer and a cheap, dependable explosive commonly used in blasting powder.
The destructive power of ammonia nitrate is apparent in photographs of the Alfred P. Murrah Federal Building in Oklahoma City, which was destroyed with a bomb made from ammonium nitrate on April 19, 1995.
Nitric acid (HNO3) and ammonia (NH3) represent the maximum (+5) and minimum (-3) oxidation numbers for nitrogen. Nitrogen also forms compounds with every oxidation number between these extremes (see table below).
Common Oxidation Numbers for Nitrogen
| Oxidation Number | Examples |
| -3 | NH3, NH4+, NH2-, Mg3N2 |
| -2 | N2H4 |
| -1 | NH2OH |
| -1/3 | NaN3, HN3 |
| 0 | N2 |
| +1 | N2O |
| +2 | NO, N2O2 |
| +3 | HNO2, NO2-, N2O3, NO+ |
| +4 | NO2, N2O4 |
| +5 | HNO3, NO3-, N2O5 |
At about the time that Haber developed the process for making ammonia and Ostwald worked out the process for converting ammonia into nitric acid, Raschig developed a process that used the hypochlorite (OCl-) ion to oxidize ammonia to produce hydrazine, N2H4.
This reaction can be understood by noting that the OCl- ion is a two-electron oxidizing agent. The loss of a pair of electrons and a pair of H+ ions by neighboring NH3 molecules would produce a pair of highly reactive NH2 molecules, which would combine to form a hydrazine molecule as shown in the figure below.
Hydrazine is a colorless liquid with a faint odor of ammonia that can be collected when this solution is heated until N2H4 distills out of the reaction flask. Many of the physical properties of hydrazine are similar to those of water.
| H2O | N2H4 | |
| Density | 1.000 g/cm3 | 1.008 g/cm3 |
| Melting Point | 0.00oC | 1.54oC |
| Boiling Point | 100oC | 113.8oC |
There is a significant difference between the chemical properties of these compounds, however. Hydrazine burns when ignited in air to give nitrogen gas, water vapor, and large amounts of energy.
| N2H4(l) | + | O2(g) | N2(g) | + | 2 H2O(g) | Ho = -534.3 kJ/mol N2H4 |
The principal use of hydrazine is as a rocket fuel. It is second only to liquid hydrogen in terms of the number of kilograms of thrust produced per kilogram of fuel burned. Hydrazine has several advantages over liquid H2, however. It can be stored at room temperature, whereas liquid hydrogen must be stored at temperatures below -253oC. Hydrazine is also more dense than liquid H2 and therefore requires less storage space.
Pure hydrazine is seldom used as a rocket fuel because it freezes at the temperatures encountered in the upper atmosphere. Hydrazine is mixed with N,N-dimethylhydrazine, (CH3)2NNH2, to form a solution that remains a liquid at low temperatures. Mixtures of hydrazine and N,N-dimethylhydrazine were used to fuel the Titan II rockets that carried the Project Gemini spacecraft, and the reaction between hydrazine derivatives and N2O4 is still used to fuel the small rocket engines that enable the space shuttle to maneuver in space.
The product of the combustion of hydrazine is unusual. When carbon compounds burn, the carbon is oxidized to CO or CO2. When sulfur compounds burn, SO2 is produced. When hydrazine is burned, the product of the reaction is N2 because of the unusually strong nitrogen-nitrogen triple bond in the N2 molecule.
Hydrazine reacts with nitrous acid (HNO2) to form hydrogen azide, HN3, in which the nitrogen atom formally has an oxidation state of -1/3.
| N2H4(aq) | + | HNO2(aq) | HN3(aq) | + | 2 H2O(l) |
Pure hydrogen azide is an extremely dangerous substance. Even dilute solutions should be handled with care because of the risk of explosions. Hydrogen azide is best described as a resonance hybrid of the Lewis structures shown in the figure below. The corresponding azide ion, N3-, is a linear molecule, which is a resonance hybrid of three Lewis structures.
Fluorine, oxygen, and chlorine are the only elements more electronegative than nitrogen. As a result, positive oxidation numbers of nitrogen are found in compounds that contain one or more of these elements.
In theory, N2 could react with F2 to form a compound with the formula NF3. In practice, N2 is too inert to undergo this reaction at room temperature. NF3 is made by reacting ammonia with F2 in the presence of a copper metal catalyst.
| Cu | |||||
| NH3(g) | + | 3 F2(g) | NF3(g) | + | 3 HF(g) |
The HF produced in this reaction combines with ammonia to form ammonium fluoride. The overall stoichiometry for the reaction is therefore written as follows.
The Lewis structure of NF3 is analogous to the Lewis structure of NH3, and the two molecules have similar shapes.
Ammonia reacts with chlorine to form NCl3, which seems at first glance to be closely related to NF3. But there is a significant difference between these compounds. NF3 is essentially inert at room temperature, whereas NCl3 is a shock-sensitive, highly explosive liquid that decomposes to form N2 and Cl2.
| 2 NCl3(l) | N2(g) | + | 3 Cl2(g) |
Ammonia reacts with iodine to form a solid that is a complex between NI3 and NH3. This material is the subject of a popular, but dangerous, demonstration in which freshly prepared samples of NI3 in ammonia are poured onto filter paper, which is allowed to dry on a ring stand. After the ammonia evaporates, the NH3/NI3 crystals are touched with a feather attached to a meter stick, resulting in detonation of this shock-sensitive solid, which decomposes to form a mixture of N2 and I2.
Lewis structures for seven oxides of nitrogen with oxidation numbers ranging from +1 to +5 are given in the table below.
These compounds all have two things in common: they contain N=O double bonds and they are less stable than their elements in the gas phase, as shown by the enthalpy of formation data in the table below.
Enthalpy of Formation Data for the Oxides of Nitrogen
| Compound | Hof (kJ/mol) |
| N2O(g) | 82.05 |
| NO(g) | 90.25 |
| NO2(g) | 33.18 |
| N2O3(g) | 83.72 |
| N2O4(g) | 9.16 |
| N2O5(g) | 11.35 |
Dinitrogen oxide, N2O, which is also known as nitrous oxide, can be prepared by carefully decomposing ammonium nitrate.
Nitrous oxide is a sweet-smelling, colorless gas best known to nonchemists as 'laughing gas.' As early as 1800, Humphry Davy noted that N2O, inhaled in relatively small amounts, produced a state of apparent intoxication often accompanied by either convulsive laughter or crying. When taken in larger doses, nitrous oxide provides fast and efficient relief from pain. N2O was therefore used as the first anesthetic. Because large doses are needed to produce anesthesia, and continued exposure to the gas can be fatal, N2O is used today only for relatively short operations.

Nitrous oxide has several other interesting properties. First, it is highly soluble in cream; for that reason, it is used as the propellant in whipped cream dispensers. Second, although it does not burn by itself, it is better than air at supporting the combustion of other objects. This can be explained by noting that N2O can decompose to form an atmosphere that is one-third O2 by volume, whereas normal air is only 21% oxygen by volume.
| 2 N2O(g) | 2 N2(g) | + | O2(g) |
For many years, the endings -ous and -ic were used to distinguish between the lowest and highest of a pair of oxidation numbers. N2O is nitrous oxide because the oxidation number of the nitrogen is +1. NO is nitric oxide because the oxidation number of the nitrogen is +2.
Enormous quantities of nitrogen oxide, or nitric oxide, are generated each year by the reaction between the N2 and O2 in the atmosphere, catalyzed by a stroke of lightning passing through the atmosphere or by the hot walls of an internal combustion engine.
One of the reasons for lowering the compression ratio of automobile engines in recent years is to decrease the temperature of the combustion reaction, thereby decreasing the amount of NO emitted into the atmosphere.
NO can be prepared in the laboratory by reacting copper metal with dilute nitric acid.
| 3 Cu(s) | + | 8 HNO3(aq) | 3 Cu(NO3)2(aq) | + | 2 NO(g) | + | 4 H2O(l) |
The NO molecule contains an odd number of valence electrons. As a result, it is impossible to write a Lewis structure for this molecule in which all of the electrons are paired (see table of oxides of nitrogen). When NO gas is cooled, pairs of NO molecules combine in a reversible reaction to form a dimer (from the Greek, 'two parts'), with the formula N2O2, in which all of the valence electrons are paired, as shown in the table of oxides of nitrogen.
NO reacts rapidly with O2 to form nitrogen dioxide (once known as nitrogen peroxide), which is a dark brown gas at room temperature.
NO2 can be prepared in the laboratory by heating certain metal nitrates until they decompose.
| 2 Pb(NO3)2(s) | 2 PbO(s) | + | 4 NO2(g) | + | O2(g) |
It can also be made by reacting copper metal with concentrated nitric acid,
NO2 also has an odd number of electrons and therefore contains at least one unpaired electron in its Lewis structures. NO2 dimerizes at low temperatures to form N2O4 molecules, in which all the electrons are paired, as shown in the table of oxides of nitrogen.
Mixtures of NO and NO2 combine when cooled to form dinitrogen trioxide, N2O3, which is a blue liquid. The formation of a blue liquid when either NO or NO2 is cooled therefore implies the presence of at least a small portion of the other oxide because N2O2 and N2O4 are both colorless.
By carefully removing water from concentrated nitric acid at low temperatures with a dehydrating agent we can form dinitrogen pentoxide.
| 4 HNO3(aq) | + | P4O10(s) | 2 N2O5(s) | + | 4 HPO3(s) |
N2O5 is a colorless solid that decomposes in light or on warming to room temperature. As might be expected, N2O5 dissolves in water to form nitric acid.
Phosphorus Number Of Electrons To Lose
Phosphorus is the first element whose discovery can be traced to a single individual. In 1669, while searching for a way to convert silver into gold, Hennig Brand obtained a white, waxy solid that glowed in the dark and burst spontaneously into flame when exposed to air. Brand made this substance by evaporating the water from urine and allowing the black residue to putrefy for several months. He then mixed this residue with sand, heated this mixture in the presence of a minimum of air, and collected under water the volatile products that distilled out of the reaction flask.
Phosphorus forms a number of compounds that are direct analogs of nitrogen-containing compounds. However, the fact that elemental nitrogen is virtually inert at room temperature, whereas elemental phosphorus can burst spontaneously into flame when exposed to air, shows that there are differences between these elements as well. Phosphorus often forms compounds with the same oxidation numbers as the analogous nitrogen compounds, but with different formulas, as shown in the table below.
Nitrogen and Phosphorus Compounds with the Same Oxidation Numbers but Different Formulas
| Oxidation Number | Nitrogen Compound | Phosphorus Compound |
| 0 | N2 | P4 |
| +3 | HNO2 (nitrous acid) | H3PO3 (phosphorous acid) |
| +3 | N2O3 | P4O6 |
| +5 | HNO3 (nitric acid) | H3PO4 (phosphoric acid) |
| +5 | NaNO3 (sodium nitrate) | Na3PO4 (sodium phosphate) |
| +5 | N2O5 | P4O10 |
The same factors that explain the differences between sulfur and oxygen can be used to explain the differences between phosphorus and nitrogen.
1. Nitrogen-nitrogen triple bonds are much stronger than phosphorus-phosphorus triple bonds.
2. P-P single bonds are stronger than N-N single bonds.
3. Phosphorus (EN = 2.19) is much less electronegative than nitrogen (EN = 3.04).
4. Phosphorus can expand its valence shell to hold more than eight electrons, but nitrogen cannot.
The ratio of the radii of phosphorus and nitrogen atoms is the same as the ratio of the radii of sulfur and oxygen atoms, within experimental error.
Phosphorus Number Of Electrons Gained Or Lost
As a result, phosphorus-phosphorus triple bonds are much weaker than Nitrogen-nitrogen triple bonds, for the same reason that S=S double bonds are weaker than O=O double bonds phosphorus atoms are too big to come close enough together to form strong bonds.
Each atom in an N2 molecule completes its octet of valence electrons by sharing three pairs of electrons with a single neighboring atom. Because phosphorus does not form strong multiple bonds with itself, elemental phosphorus consists of tetrahedral P4 molecules in which each atom forms single bonds with three neighboring atoms, as shown in the figure below.
Phosphorus is a white solid with a waxy appearance, which melts at 44.1oC and boils at 287oC. It is made by reducing calcium phosphate with carbon in the presence of silica (sand) at very high temperatures.
| 2 Ca3(PO4)2(s) | + | 6 SiO2(s) | + | 10 C(s) | 6 CaSiO3(s) | + | P4(s) | + | 10 CO(g) |
White phosphorus is stored under water because the element spontaneously bursts into flame in the presence of oxygen at temperatures only slightly above room temperature. Although phosphorus is insoluble in water, it is very soluble in carbon disulfide. Solutions of P4 in CS2 are reasonably stable. As soon as the CS2 evaporates, however, the phosphorus bursts into flame.
The P-P-P bond angle in a tetrahedral P4 molecule is only 60o. This very small angle produces a considerable amount of strain in the P4 molecule, which can be relieved by breaking one of the P-P bonds. Phosphorus therefore forms other allotropes by opening up the P4 tetrahedron. When white phosphorus is heated to 300oC, one bond inside each P4 tetrahedron is broken, and the P4 molecules link together to form a polymer (from the Greek pol, 'many,' and meros, 'parts') with the structure shown in the figure below. This allotrope of phosphorus is dark red, and its presence in small traces often gives white phosphorus a light yellow color. Red phosphorus is more dense (2.16 g/cm3) than white phosphorus (1.82 g/cm3) and is much less reactive at normal temperatures.
The size of a phosphorus atom also interferes with its ability to form double bonds to other elements, such as oxygen, nitrogen, and sulfur. As a result, phosphorus tends to form compounds that contain two P-O single bonds where nitrogen would form an N=O double bond. Nitrogen forms the nitrate, NO3-, ion, for example, in which it has an oxidation number of +5. When phosphorus forms an ion with the same oxidation number, it is the phosphate, PO43-, ion, as shown in the figure below.
Phosphorus Number Of Electrons And Protons
Similarly, nitrogen forms nitric acid, HNO3, which contains an N=O double bond, whereas phosphorus forms phosphoric acid, H3PO4, which contains P-O single bonds, as shown in the figure below.
The difference between the electronegativities of phosphorus and nitrogen (EN = 0.85) is the same as the difference between the electronegativities of sulfur and oxygen (EN = 0.86), within experimental error. Because it is less electronegative, phosphorus is more likely than nitrogen to exhibit positive oxidation numbers. The most important oxidation numbers for phosphorus are -3, +3, and +5 (see table below).
Common Oxidation Numbers of Phosphorus
| Oxidation Number | Examples |
| -3 | Ca3P2, PH3 |
| +3 | PF3, P4O10, H3PO3 |
| +5 | PF5, P4O10, H3PO4, PO43- |
Because it is more electronegative than most metals, phosphorus reacts with metals at elevated temperatures to form phosphides, in which it has an oxidation number of -3.
These metal phosphides react with water to produce a poisonous, highly reactive, colorless gas known as phosphine (PH3), which has the foulest odor the authors have encountered.
| Ca3P2(s) | + | 6 H2O(l) | 2 PH3(g) | + | 3 Ca2+(aq) | + | 6 OH-(aq) |
Samples of PH3, the phosphorus analog of ammonia, are often contaminated by traces of P2H4, the phosphorus analog of hydrazine. As if the toxicity and odor of PH3 were not enough, mixtures of PH3 and P2H4 burst spontaneously into flame in the presence of oxygen.
Compounds (such as Ca3P2 and PH3) in which phosphorus has a negative oxidation number are far outnumbered by compounds in which the oxidation number of phosphorus is positive. Phosphorus burns in O2 to produce P4O10 in a reaction that gives off extraordinary amounts of energy in the form of heat and light.
When phosphorus burns in the presence of a limited amount of O2, P4O6 is produced.
| P4(s) | + | 3 O2(g) | P4O6(s) | Ho = -1640 kJ/mol P4 |
P4O6 consists of a tetrahedron in which an oxygen atom has been inserted into each P-P bond in the P4 molecule (see figure below). P4O10 has an analogous structure, with an additional oxygen atom bound to each of the four phosphorus atoms.
P4O6 and P4O10 react with water to form phosphorous acid, H3PO3, and phosphoric acid, H3PO4, respectively.
| P4O6(s) | + | 6 H2O(l) | 4 H3PO3(aq) |
| P4O10(s) | + | 6 H2O(l) | 4 H3PO4(aq) |
P4O10 has such a high affinity for water that it is commonly used as a dehydrating agent. Phosphorous acid, H3PO3, and phosphoric acid, H3PO4, are examples of a large class of oxyacids of phosphorus. Lewis structures for some of these oxyacids and their related oxyanions are given in the table below.
The reaction between ammonia and fluorine stops at NF3 because nitrogen uses the 2s, 2px, 2py and 2pz orbitals to hold valence electrons. Nitrogen atoms can therefore hold a maximum of eight valence electrons. Phosphorus, however, has empty 3d atomic orbitals that can be used to expand the valence shell to hold 10 or more electrons. Thus, phosphorus can react with fluorine to form both PF3 and PF5. Phosphorus can even form the PF6- ion, in which there are 12 valence electrons on the central atom, as shown in the figure below.
